
Understanding the formation of Universe to resolve the Energy
Crisis.
Cosmologists are
agreed that the universe began with a big bang. Evidence comes from the fact
that the universe is still expanding today, with clusters of galaxies flying
apart from each other at immense speeds. If the universe is expanding, it had
to be smaller in the past.
According to the most recent measurements and
observations , the time the Big Bang occurred and the age of the Universe, is
calculated as approximately 13.7 billion years.
Brief about Big Bang, nucleosynthesis, and the modern search of
formation of Structure of matter.
Ø
In the beginning there was nothing and then Big Bang
occurred. In first seconds the temperature was billions of degrees. The Cosmic soup expanded very fast and then rouhghly three
minutes after Big Bang, started cooling to form photons, bosons,gluons,
gravtions ,energy radiation discussed below.
Ø Temperatures further dropped and the forces devided.
Up, down, top, charmed and strange quarks together with glue balls and chain to
form protons and neutrons, mesons and leptons, baryons and bosons
Now on Earth, Big Bang experiments are carried out in the
Laboratory Particle Accelerators and experimenters have managed to recreate the
extra ordinarily hot, dense medium on Earth.
Ø Gravity started to form hydrogen and helium gas,
coalesce to form the giant clouds that became first galaxies and stars.
High density and hot
temperature of early Big Bang could produce light elements Hydrogen, Isotope Deuterium, Helium, small
amount of Isotope tritium, Lithium, via fusion.
Ø After next half hour,
Universe cooled and expanded to point where fusion of heavier elements was
impossible. Additional Heavier Elements
originate in interiors of very first stars relatively late in Universe's
history.
Related Video:
The
plutonium isotopes plutonium-244 and plutonium-239 have also been found
in trace amounts on earth(almost all plutonium-239 and other heavier elements)
thought to be consumed to form lower elements/ isotops in the early period of Supernova
explosion when the temperature of the
universe began to drop but still was very high for fast decomposition. Since
1940, 26 new elements beyond Uranium have been synthesized in laboratories and added
to the periodic table.
"These
new elements expand our understanding of the universe and provide important
tests of nuclear theories.
The Cosmic Calendar
Primary goal in modern physics is to answer the question
"What is the Universe made of?" Often that question reduces to
"What is matter and what holds it together?"
Modern physics and particle physics
speak of fundamental building blocks of Nature, where fundamental (elementary) means simple and structure less.
The search for the origin of matter means the understanding
of elementary particles, which requires
an understanding of not only their characteristics, but how they interact and
relate to other particles and forces of Nature.
Brief history of searching the Fundamental building blocks of
matter.
History of Atom and Elements:
Related video
Quantum Mechanics:
Related
video
Modern Concepts of Elementary Particles:
Fundamental or Elementary particles are particles with no measurable internal structure;
that is, they are not composed of other particles. They are the fundamental
objects of quantum field theory.
More than 200 subatomic
particles have been discovered so far, all detected in sophisticated particle
accelerators. However, most are not fundamental, most are composed of other,
simpler particles.
Many families and sub-families of elementary particles
exist. Elementary particles are classified according to their spin.
Present Standard Model of
particle physics lays out the properties of all known elementary particles and
describes three of the four fundamental forces that govern nature accept Gravitons.
Quarks and Leptons:
The two most fundamental types of particles
are quarks and lepton. Quarks and
leptons are also called Fermions( Observed in Fermi lab.)
Fermions comprise
all particles with spin of 1/2.
1) Leptons:
Electrons are part of a particle family called leptons
LEPTONS
Abbrev Elec Charge Mass
Electron e -1 0.511 MeV Stable
Muon u -1 105 MeV Unstable
Tau T -1 1.78 GeV Unstable.
Electron e -1 0.511 MeV Stable
Muon u -1 105 MeV Unstable
Tau T -1 1.78 GeV Unstable.
There are three charged
leptons—electrons, muons, and taus, all with a negative electric charge. The
muon is about 200 times more massive than the electron; the tau is a whopping
3000 times heavier than the electron. Muon
and Tau aren't stable. means they live only a short time before they decay into
lighter particles. Muon-to-electron conversion at high energies is a sign of
the existence of new particles.
There are three Neutrinos corresponding to each of the above three
leptons. Leptons come in pairs, each neutrono has a charged partner. They are
referred to as electron neutrino, muon neutrino and tau neutrino. Neutrinos
have no charge and rarely interact with ordinary matter. They were long assumed
to be mass less.
Recent neutrino experiments have shown,
however, that neutrinos have a tiny mass, much smaller than that of electron: m
electron neutrino < 0.0000059 m electron.
They were first created in the Big Bang, in
the beginning of the Universe, and continue to be created in nuclear reactions
in supernovae, or in fusion reactions inside stars like the Sun. Neutrinos are
also created in the interaction of cosmic rays with the atmosphere of Earth and
emitted in the radioactive decay of elements inside the Earth.
Beside this, man-made neutrinos are
produced in nuclear reactions inside nuclear power plants and in particle
interactions at accelerators like at Fermi lab. They travel through the
Universe with a speed close to the speed of light.
In free space or through the additional
interaction with matter, neutrinos have the ability to spontaneously change
their type.
The periodic change of neutrino flavor
from one type into another is known as neutrino
oscillation.
Despite their small contribution to the
overall content of the Universe, neutrinos play a crucial role in the evolution
of the Universe. Freely streaming through the cosmose they affect the formation
of large-scale structures in the Universe, play a central role in the energy
release of supernovae, and are central to nuclear decays, and particle interactions.
Related Video:
2) Quarks:
Fundamental particles Quarks combine to form the composite particles of matter, Hadrons.
QUARKS
Abbrev Elec Charge Mass
Up u +2/3 2 MeV Stable
Down d -1/3 5 MeV Stable
Up u +2/3 2 MeV Stable
Down d -1/3 5 MeV Stable
Two Up
quarks and 1 Down quark make a Proton with net charge of +1.
Two Down quarks and 1 Up quark make a Neutron with net charge of 0.
Charm C +2/3 1.25 GeV Unstable
Strange S - 1/3 95 MeV Unstable
Top t +2/3 171 GeV Unstable
Bottom b -1/3 4.2 GeV Unstable
Two Down quarks and 1 Up quark make a Neutron with net charge of 0.
Charm C +2/3 1.25 GeV Unstable
Strange S - 1/3 95 MeV Unstable
Top t +2/3 171 GeV Unstable
Bottom b -1/3 4.2 GeV Unstable
The
unstable quarks make up short-lived particles, seen only in very high energy
physics labs and cosmic rays.
Hadrons are subdivided into two catagories:
* Baryons (protons and neutrons)
* Mesons (pions and kaons)
Baryons are made of three quarks to form the protons and neutrons of atomic
nuclei (and also anti-protons and anti-neutrons).
Mesons mentioned below, made of quark pairs (two quarks), are usually found in cosmic rays. All the quarks combine to make
charges of -1, 0, or +1.
Matter
is effected by forces or interactions (the terms are interchangeable). There
are four fundamental forces in the Universe:
1)
gravitation (between particles with mass)
2) weak nuclear force (operates between
neutrinos and electrons)
3)
electromagnetic (between particles with charge/magnetism)
4)
strong nuclear force (between quarks)
Related Video:
3) Bosons (force carriers):
Bosons carry the forces that act to bind or attract
particles. Force carriers are not
considered matter. Bosons do not obey the Pauli exclusion principle which says
“no two particles in
the same quantum state could exist in the same place at the same time”.
Graviton:
The
Graviton is the boson that is believed to act as the exchange particle for the
gravitational force.
Except
for the, graviton, Higgs boson and dark matter, all the force carrier particles
mentioned so far have been discovered or proven to exist in the laboratory.
The Higgs Boson and Mass
Weak Force (W and Z Bosons):
The Z boson, W- boson, and W+ boson operate over very tiny
inter-atomic distances (10^-18 meters), carrying the weak force.
Photon: (The Electromagnetic Force carrier
Bosons)
Photons have zero mass, as
far as we know, and always travel at the "speed of light", c, which
is about 300,000,000 meters per second, or 186,000 miles per second, in a
vacuum.
Photons are the most obvious bosons.They
are the carrier of electromagnetic radiations of
different energies span (eg: light, radio, television, gamma rays, X-rays).
Photons can have an effect over huge distances. Photons can behave as particles
or waves, leading to a duality that underlies much of quantum physics.
Gluon:
Gluons
come in eight different species. Quarks have electromagnetic charge, and they also have an
altogether different kind of charge called color charge. The force between color-charged particles is very
strong, so this force is "creatively" called - strong force.
The strong force holds quarks together to form hadrons(protons or neutrons), so
its carrier particles are whimsically called gluons because they so tightly
"glue" quarks together.
Force carriers are not
considered matter: though carriers of the electric force (photons) possess
energy and the carriers of the weak force (W and Z bosons) are
massive, but neither are considered matter either.
However, while these
particles are not considered matter, they do contribute to the total mass of
atom, subatomic particles and all systems which contain them.
Review of above discussion of FERMIONS & BOSONS:
Related
Video:
PROPERTIES OF THE INTERACTIONS OF VARIOUS FORCES
ON VARIOUS PARTICLES.
Particle
accelerators allow physicists to look farther and farther back jn time, to
revisit the ultra high energies of the early Universe after the Big Bang. Do
the four forces that dictate the interactions of particles converge to a single
“Grand Unified Force” at ultra-high energy?
Progress
is being made toward combining the Strong Nuclear Force with the Electroweak
Force, but as yet how to include Gravity remains
a problem.
Understanding of Present Standard Model of Atom and interaction of
various forces on Elementry particles within the Atom in view of the above
discussion.
The protons and neutrons collectively present in the nucleus
of an atom are called nucleons.
Color strong force (gluon) is
the nuclear force that acts between the three quarks that
a proton or neutron is
made of. Strong force has three types of charges which are named after three
basic colors, they are red, blue, and green.
Quarks
are affected by gluons. A gluon has the ability to change the color (also
called flavor) of a quark in order to keep the overall color charge of the
baryon neutral (red light + blue light + green light = white light).
If
the gluons that held quarks in the proton disappear, quarks would fall out of
the proton. The
force between two quarks or between a quark and an antiquark, mediated by gluons, is about 100 times stronger than the
conventional nuclear force.
What binds the nucleus together?
The nucleus of an atom
consists of protons and neutrons crammed together. Since neutrons have no
charge and the positively-charged protons repel one another, why doesn't the nucleus blow apart?
Protons and neutrons emit and absorb mesons (which are made of one quark and one antiquark), giving
rise to the nuclear force that binds the nucleus of an atom.
What binds the nucleus of two or more atoms:
Atoms usually have the same numbers of protons and electrons.
They are electrically neutral, therefore, because the positive protons cancel
out the negative electrons. Since they are neutral, what causes them to stick
together to form stable molecules of an element?
1) The strong force
between the quarks in one proton and the quarks in another proton is strong
enough to overwhelm the repulsive electromagnetic force.
2) It has
been discovered that the charged parts of one atom can interact with the
charged parts of another atom of element as shown above.This allows different
atoms to bind together, an effect called the residual
electromagnetic force, which allows the similar
atoms to bond and form molecules.
The above mentioned combined nuclear forces cause the nucleus of two or more atoms to stick
together to form stable molecules.
So Nuclear forces are
attractive forces which exist in between protons and neutrons, neutron and
neutron, proton and proton , electron and proton of other. It is the strongest
force. It is much stronger than gravitational and electrostatic forces
due to positive charge on protons. As discussed above, this strong force is
about 100 times stronger than the
electromagnetic force, but it only operates on extremely tiny distances, i.e.
the scale of nucleons.
However, when its power is released, by breaking or fusing together atomic
nuclei, the results are incredible as in the case of atomic bombs, or the Sun,
which both operate by manipulating nucleons.
This is generally known as "Nuclear energy."
Related
videos:
VIDEO-1
VIDEO-II
NUCLEUS INSTABILITY:
The nucleus of an atom contains protons and
neutrons held together by the strong nuclear force. However, when there are too
many or too few neutrons in the nucleus, it becomes unstable. Beta decay occurs
when the ratio of neutrons to protons is too great, or too small. Neutrons can
emit an electron and become a proton to stabilize this ratio. Protons can also
emit a positron and become a neutron. This results in the original atom
becoming a different element because the number of protons change.
Observations from the above plot:
All the stable nuclei
lie within a definite area called the Zone of stability.
i) For low atomic
numbers most stable nuclei have a neutron/proton ratio which is very close to
1. As the atomic number increases, the zone of stability corresponds to a
gradually increasing neutron/proton ratio. In the case of the heaviest stable
isotope, for instance, the neutron/proton ratio is 1.518. If an unstable
isotope lies to the left of the zone of stability in Fig., it is neutron rich
and decays by β emission. If it lies to the right of the zone, it is proton rich and
decays by positron emission or electron capture.
ii) Another factor
affecting the stability of a nucleus is whether the number of protons and
neutrons is even or odd. Among the 354 known stable isotopes, 157 (almost half) have an even number of protons and
an even number of neutrons. Only five have an odd number of both kinds of
nucleons. In the universe as a whole (with the exception of hydrogen) we find
that the even-numbered elements are almost always much more abundant than the
odd-numbered elements close to them in the periodic table.
iii) Finally there is
a particular stability associated with nuclei in which either the number of
protons or the number of neutrons is equal to one of the so-called "magic" numbers 2, 8, 20,
28, 50, 82, and 126. Of particular
stability, and also of high abundance in the universe, are nuclei in which both
the-number of protons and the number of neutrons correspond to magic numbers.
Nuclear energy:
Nuclear
energy is released by three exothermic processes:
i.
Radioactive
decay, is the spontaneous
disintegration of atomic nuclei by emitting either alpha particles, Beta
particles, gamma rays, (or all of them)
Radioactive decay
All
radioactive elements (unstable) began to decay as soon as they had been created
in supernova or stellar nucleosynthesis in early high temperatures and pressure
conditions.
Understanding of Radiations and Radioactive Decay:
The
four most common modes of radioactive decay are: alpha decay, beta decay, inverse beta decay
(considered as both positron
emission
and electron capture),
and isomeric transition.
nuclei).
The
step-by-step process by which radioactive elements emit alpha, Beta and gamma
radiations, change into other elements and finally reach stability is called a
decay series.
The time involved in radioactive decay is
known as half-life (the length of time it takes for one-half of a given number
of atoms of one element to decay into another element). The half lives of the
isotopes formed in a decay chain may be measured in seconds, minutes, days,
years, or millennia which help us in estimation of geological age of rocks.
Thorium-232,
Uranium-238, and Uranium-235 have existed since the formation of the earth.
The
plutonium isotopes( Pu-244 and Pu-239)
have also been found in trace amounts on earth(almost all plutonium-239 and
other heavier elements) thought to be consumed to form lower elements/isotopes
in the early period of big bang when the temperature of the universe began to
drop but still was very high for fast decomposition.
Three main
decay chains (or families) are observed in nature on Earth, commonly called the
radium (Uranium)
series, the thorium
series, and the actinium
series and ending in three different, stable isotopes of Lead (206,208,207).
Mentioned below is the graph of binding
energy which plots the binding energy per nucleon against atomic mass. This
curve has its main peak at iron and then slowly decreases again and also a
narrow isolated peak at helium, which as noted is very stable.
The heaviest nuclei in nature, uranium 235U & 238U, are unstable, but having a
lifetime of 4.5 billion years for 235U, close to the age of the Earth and
longer for 238U, they are still relatively abundant; they (and other nuclei
heavier than iron) may have formed in a supernova explosion , preceding the
formation of the solar system. The most common isotope of thorium, 232Th,
also undergoes α particle emission, and its half-life (time over which half a number of
atoms decays) is even longer, by several times. In each of these, radioactive
decay produces daughter isotopes which are also unstable, starting a chain of
decays which ends in some stable isotope of lead.
Binding Energy per nucleon plotted as a function of atomic mass
number.
Observations from Average binding energy plot.
(i) Binding energy per nucleon increases from 1.1 to 8.0 MeV
from mass number 2 to 20.
(ii) Binding energy per nucleon increses from 8 to 8.6 MeV from mass number 20 to 40.
(iii) Binding energy per nucleon remains 8.6 – 8.7 MeV from mass number 40 to 90. Iron (56) has the maximum value of 8.7 MeV per nucleon.
(iv) The value of binding energy per nucleon decreases from 8.6 to 7.5 MeV from mass number 90 to 240.
(v) Points for helium, carbon, oxygen lie quite high in the graph showing that these nuclei are highly stable.
(ii) Binding energy per nucleon increses from 8 to 8.6 MeV from mass number 20 to 40.
(iii) Binding energy per nucleon remains 8.6 – 8.7 MeV from mass number 40 to 90. Iron (56) has the maximum value of 8.7 MeV per nucleon.
(iv) The value of binding energy per nucleon decreases from 8.6 to 7.5 MeV from mass number 90 to 240.
(v) Points for helium, carbon, oxygen lie quite high in the graph showing that these nuclei are highly stable.
(vi) A notable exception to this general trend is
the helium-4 nucleus, whose
binding energy is higher than that of lithium,
the next heaviest element. The Pauli exclusion principle provides an
explanation for this exceptional behavior—it says that because protons and
neutrons are fermions,
they cannot exist in exactly the same state. Each proton or neutron energy
state in a nucleus can accommodate both a spin up particle and a spin down
particle. Helium-4 has an anomalously large binding energy because its nucleus
consists of two protons and two neutrons; so all four of its nucleons can be in
the ground state.
(vii) At
the peak of binding energy, nickel-62 is the most tightly
bound nucleus (per nucleon), followed by iron-58 and iron-56.
Nuclear Reactions are regulated by the Nuclear Binding Energy.
Protons and neutrons are held together by the
strong force, which only acts over very small distances as discussed above but
is able to overcome the electrostatic repulsion between protons. The strength
of the bonding is measured by the binding energy per nucleon where “nucleon” is
a collective name for neutrons and protons. But the total mass of the nucleus
is less than the sum of the mass of the individual neutrons and protons that formed it.
The difference in mass is equivalent to the energy released
in forming the nucleus of an atom and known as the mass
defect per nucleon.
Example of Helium Nucleus :
The mass of a proton is 1.00728 atomic mass units (u),
while neutrons weigh 1.00866 u.
The alpha particle (helium nucleus)
has less mass than the sum of the masses of the individual particles that make
it up.
When the four nucleons combine,
the extra mass is transformed into the energy that holds them together in the
nucleus of the atom. The mass can be directly converted to energy, the binding energy of the nucleus as per Einstein's equation.
It is also the energy required to break (unbind) a nucleus into separate protons and neutrons.
The attractive nuclear force
(strong nuclear
force), which binds protons and
neutrons equally to each other, has a limited range due to a rapid exponential
decrease in this force with distance as shown in figure below and also discussed
above.
The
general decrease in binding energy beyond iron is due to the fact
that, as a nucleus gets bigger, the ability of the strong force to counteract
the electrostatic repulsion between protons becomes weaker.
For both the smaller and more massive
nuclides, the stability is less. This leads to some interesting reactions.
The most tightly bound isotopes are 62Ni, 58Fe, and 56Fe, which have binding energies of 8,8 MeV per
nucleon. Elements heavier than these isotopes can yield energy by nuclear fission; lighter isotopes can yield energy by fusion.
In fusion and fission nuclear reactions, nuclear
energy produces thermal energy, which is given off as heat.
Fusion Reaction:
Fusion is the production of heavier elements by the fusing of
lighter elements.
Tremendous Nuclear energy inside hydrogen
nuclei is released, when hydrogen nuclei fuse to form a helium nucleus. The sun
and other stars use the fusion to generate radiant and thermal energy.
Here on Earth, future fusion
plants will imitate the Sun, fusing deuterium and tritium atoms at temperatures
over 100 million degrees K, releasing energy for a variety of uses, including
electricity. Such a condition where the
thermal energy of nuclei is high enough to fuse despite their repulsion is
called thermonuclear. The fuel for this fusion
is found in water, and can therefore provide energy for the world for billions
of years. Progress in fusion research indicates fusion to be a practical energy
source sometime in the 21st century (Ref:
Experiment at RIKEN–RAL Muon Facility Japan & UK, mentioned below).
Fission Reaction:
Nuclear energy is released during atomic
fission, when uranium nuclei are split. Fission's heat is used to generate
electric power in hundreds of locations worldwide.
Atoms
of the same element with a different number of neutrons are called isotopes. The isotope of uranium that is needed for
nuclear fission, and therefore, nuclear energy, is Uranium-235.
This
isotope is unique because it can undergo induced fission, which means its
nucleus can be forced to split. This happens when a thermal neutron runs into
the nucleus of U-235, which absorbs the neutron, becomes unstable, and breaks
into two nuclei of
lighter elements. In the process, two or three
neutrons are also released, which further collide with other U-235 atoms,
causing a huge chain reaction. The amount of energy released is incredible- a
pound of highly enriched uranium has about the same energy as a million gallons
of gasoline.
Both fission and fusion
nuclear reactions release energy by converting some of the nuclear mass into
gamma-rays, this is the famous formulation by Einstein E=mc2 as discussed
above.
Related
video:
PRESENTLY TWO IMPORTANT EXPERIMENTS ARE IN PROGRESS BASED ON PARTICLE
PHYSICS.
i) Experimenting with Neutrino.
ii) Experimenting Muon Catalyzed fusion for
energy production.
i) Experimenting with NeutrinoS:
In a recent report of June 27, 2011, Fermi
Lab US Discovers Clues to Why the Big Bang Produced More Matter than Antimatter.
Scientists of the MINOS experiment at the
Department of Energy’s Fermi National Accelerator Laboratory US have announced
the results from a search for a rare phenomenon, the transformation of muon
neutrinos into electron neutrinos. The result is consistent with and
significantly constrains a measurement reported 10 days ago by the Japanese T2K
experiment, which could have implications for understanding of the role that
neutrinos may have played in the evolution of the universe. If muon neutrinos
transform into electron neutrinos, neutrinos could be the reason that the big
bang produced more matter than antimatter, leading to the universe as it exists
today.
The Daya Bay Reactor Neutrino
Experiment China:
Source: Daya Bay Reactor Neutrino Experiment
Content: Press Release
Date Issued: 8 March 2012
Content: Press Release
Date Issued: 8 March 2012
From its beginnings in 2006, the Daya Bay Reactor Neutrino
Experiment has established new scientific milestones as the first equal
partnership between the U.S. and China in a major physics project.
Initial U.S. participation was guided by James Siegrist,
then Associate Laboratory Director for General Sciences and Director of the
Physics Division at the U.S. Department of Energy’s Lawrence Berkeley National
Laboratory (Berkeley Lab).
Discovery
of a New Kind of Neutrino Transformation.
The Daya Bay Reactor Neutrino Experiment, a
multinational collaboration operating in the south of China, today reported the
first results of its search that neutrinos can appear to vanish as they travel?
. The
Guangdong Daya Bay Nuclear Power Station, where neutrinos are born.
Traveling at close to the speed of light, the
three basic neutrino "flavors" - electron, muon, and tau neutrinos,
as well as their corresponding antineutrinos - mix together and oscillate
(transform), but this activity is extremely difficult to detect. From Dec. 24, 2011,
until Feb. 17, 2012, scientists in the Daya Bay collaboration observed tens of
thousands of interactions of electron antineutrinos, caught by six massive
detectors buried in the mountains adjacent to the powerful nuclear reactors of
the China Guangdong Nuclear Power Group. These reactors, at Daya Bay and nearby
Ling Ao, produce millions of quadrillions of elusive electron antineutrinos
every second.
Neutrinos,
the wispy particles that flooded the universe in the earliest moments after the
big bang, are continually produced in the hearts of stars and other nuclear
reactions. Untouched by electromagnetism, they respond only to the weak nuclear
force and even weaker gravity, passing mostly unhindered through everything
from planets to people. The challenge of capturing these elusive particles
inspired the Daya Bay collaboration in the design and precise placement of its
detectors.
Each antineutrino detector at Daya Bay is lined with
photomultiplier tubes to catch the faint trace of antineutrino reactions in the
scintillator fluids that fill the detectors. (Photo Roy Kaltschmidt, Lawrence
Berkeley National Laboratory)
The
plentiful data revealed for the first time the strong signal of the effect that
the scientists were searching for, a so‑called “mixing
angle” named theta one-three (written θ13), which the
researchers measured with unmatched precision. Theta one-three, the last mixing
angle to be precisely measured, expresses how electron neutrinos and their
antineutrino counterparts mix and change into the other flavors. The Daya Bay
collaboration’s first results indicate that sin2 2 θ13 is equal to 0.092(
plus or minus)0.017.
This is a new type of neutrino oscillation,
and it is surprisingly large,” says Yifang Wang of China’s Institute of High
Energy Physics (IHEP), co-spokesperson and Chinese project manager of the Daya
Bay experiment. “Our precise measurement will complete the understanding of the
neutrino oscillation and pave the way for the future understanding of
matter-antimatter asymmetry in the universe.”
ii) EXPERIMENTING Muon Catalyzed fusion for energy production:
Muon
research at the RIKEN–RAL Muon Facility Japan & UK could lead to
commercially viable fusion technology for clean energy generation.
The Rutherford-Appletion Laboratory UK (left) and the
RIKEN-RAL Moun Facility Japan (right).
In the Sun's core, hydrogen nuclei move violently due
to the extreme temperature, and the ultra high density resulting from the Sun's
massive gravity causes the hydrogen nuclei to be forced together to within one
ten-trillionth of a centimeter, inducing a chain of nuclear fusions.
Director of the
RIKEN-RAL Muon Facility. Teiichiro Matsuzaki and scientists at the facility have
been conducting unique experiments as part of fundamental research into the use
of muons to develop industrially viable nuclear fusion technology.
To achieve nuclear fusion on Earth, Deuterium (d) and tritium
(t) nuclei are used as the fuel in place of hydrogen, as these nuclei are more
readily induced into nuclear fusion and the reaction releases greater energy.
Whereas a hydrogen nucleus consists of just one proton, a deuterium nucleus
consists of one proton and one neutron, and a tritium nucleus consists of one
proton and two neutrons.
Muon-based nuclear fusion is conducted using negative muons.
A mixed gas of deuterium and tritium is cooled to temperatures below around −250°C, causing the gas to form a liquid or solid. The
injection of a beam of muons (µ) into the medium then generates muonic tritium
atoms (tµ), which are similar to hydrogen atoms. As muons are 207 times heavier
than electrons, the muon orbits the nucleus at a distance much shorter than
that for electrons. Thus, tµ atoms are extremely small, and because the tµ
atoms have no charge, they collide with deuterium atoms without being affected
by repulsive electrical force. This process produces muonic deuterium–tritium
molecules (dtµ), which are also similar to hydrogen atoms, and which have a
nucleus consisting of a muon, a deuterium nucleus and a tritium nucleus.
Similar to the tµ atom, the dtµ molecule is extremely small, which allows the
deuterium and tritium nuclei to come into very close proximity, thus inducing
d–t nuclear fusion as shown in the above figure.
Until 5–10 years ago, muon-catalyzed nuclear fusion
experiments were conducted in muon facilities in the United States, Switzerland
and Russia, and all achieved an energy balance of about 40%. These countries
withdrew from these studies because of the decommissioning of muon facilities
or a lack of experts who could deal with tritium, a radioactive material.
Director
of RIKEN –RAL Teiichiro Matsuzaki says, RIKEN-RAL Muon Facility, now
the only research institute in the world that continues to perform fundamental
experiments on muon-catalyzed nuclear fusion, to achieve scientific break-even
conditions in an effort to put nuclear fusion into practical use for energy
production.
Suggestions:
Ø Need to join the research work being carried out on energy projects in
friendly countries
to get acquainted with the new
technologies coming up for energy production.
Ø Need
to upgrade our Universities to international standards and provide research
facilities with proper funding.
Ø
Need to boost the private sector as a driving force to invest
in clean and renewable energy technologies in Pakistan.





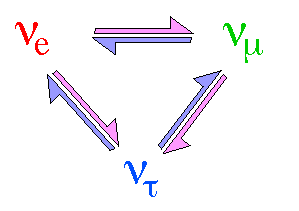
















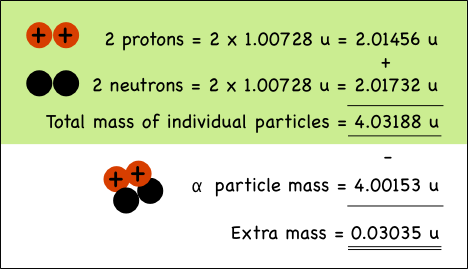








No comments:
Post a Comment
Note: Only a member of this blog may post a comment.